14+ Chapter 7 Study Guide Ionic Compounds And Metals
Web Ionic Character and Electronegativity. Due to the advancement in this area new concepts such as models of bonding molecular structure important insights on the functioning of complex components of the biological system etc.
Ionic Compounds Barbaraelam Rice
Atoms have different atomic and ionic radii.

. Amorphous solids are solids without a regulardefinitive arrangement of its constituent particles ions atoms or molecules and they possess something called the short-range order ie a regular and periodically repeating arrangement is seen only over short. Conduct electricity when melted. The empty string is the special case where the sequence has length zero so there are no symbols in the string.
An atom can become an ion if it gains or loses an electron and becomes positively or negatively charged. NCERT Solutions for Class 10 Science provides solutions to all the questions asked at the end of every chapter and. Ionic compounds are solids that typically melt at high temperatures and boil at even higher temperatures.
Each subshell s p d f has a specific number of. They float free as though floating through a sea of electrons. December 2 2022 4.
Sanlams Business Combination With Absa. 714 Decline in. An ionic compound always contains an equal magnitude of positive and negative charges.
Web Here Z is an ionic compound and Ionic compounds have melting point hence option b is a wrong statement. Web Iron ˈ aɪ ə n is a chemical element with symbol Fe from Latin. They roam around the whole metal complex.
NCERT Solutions for Class 10 Science Chapter 3 Metals and Non-Metals helps students in learning concepts given in the textbook in detail. The electronic configurations of three elements X Y and Z are X 2 8. Web Join an activity with your class and find or create your own quizzes and flashcards.
Web We would like to show you a description here but the site wont allow us. C a C l 2 N a C l K 2 S O 4 etc. Be - Mg - Sr - Ca Rank from largest to smallest radius Atomic Radii and Effective Nuclear Charge Part BRank the following.
Web The course content outlined below is organized into commonly taught units of study that provide one possible sequence for the course. December 1 2022 4. The nucleus is made of one or more protons and a number of neutronsOnly the most common variety of hydrogen has no neutrons.
Y 2 8 7 and Z 2 8 2. Web December 14 2022 4. Web Every atom is composed of a nucleus and one or more electrons bound to the nucleus.
Properties of Ionic Compounds. These Metals and Non-Metals Class 10 MCQs have been introduced in the board exam to test students analytical and reasoning skills. Solutions A nd Solution Stoichiometry 71 Introduction 72 Types of Solutions.
Though MCQs seem to be easy students need to have a good hold of the concepts to answer them. MTN Nigeria Communications PIcs 100 Million Financing Round. With some exceptions those in nonmetals are fixed in place resulting in nonmetals.
Web You can often recognize ionic compounds because of their properties. Web Shapes of Orbitals. Molecular and Ionic Compound Structure and Properties Youll discover the range of chemical bonds and how their structure can affect the properties of the.
So BeSO 4 is more soluble than ionic BaSO 4. Egypts Contract for the Operation and Maintenance First High-Speed Rail Network. Web Covalent compounds like beryllium sulphate have a higher enthalpy of dissociation than ionic barium sulphate.
NCERT Solutions for Class 10 Science provides answers to all the questions printed at the end of every chapter as well as the. Just like each home has its own design and layout electrons reside in orbitals that have their own unique shapes. Atoms are extremely small typically around 100 picometers.
Web NCERT Solutions for Class 10 Science Chapter 3 CBSE Free PDF Download. Web NCERT Solutions for Class 10 Science Chapter 4 CBSE Free PDF Available. Give a few examples of amorphous solids.
Web Study with Quizlet and memorize flashcards containing terms like Part 1Atomic Radii and Effective Nuclear Charge Atomic Radii and Effective Nuclear Charge Part A. To determine when bonds are ionic polar covalent or non-polar covalent these options are known as ionic character it is essential to have a grasp on the. NCERT Solutions for Class 10 Science Chapter 4 Carbon and Its Compounds helps students to understand concepts provided in the textbook in detail.
Web Ionic Radius. Define the term amorphous. They range from colorless gases like hydrogen to shiny solids like carbon as graphiteThe electrons in nonmetals behave differently from those in metals.
As you read about covalent and ionic compounds in Chapters 3 and 4 you learned that ionic compounds have the highest polarity forming full cations and anions within each molecule as electrons are donated from one atom to another. But the smaller entities like beryllium have higher charge density resulting in higher solvation and hence release of hydration enthalpy larger than the dissociating energy. It is a metal that belongs to the first transition series and group 8 of the periodic tableIt is by mass the most common element on Earth right in front of oxygen 321 and 301 respectively forming much of Earths outer and inner coreIt is the fourth most.
As a comparison the molecular compound water melts at 0 C and boils at 100 C. The radius of a neutral atom is. Valence electrons and ionic compounds.
Ionic compounds are chemical compounds in which oppositely charged ions are held together by electrostatic forces called ionic bonds. Web Coordination Compounds Class 12 Notes Chapter 9 Coordination compounds is a challenging area in modern inorganic chemistry. The electrostatic attractions between the oppositely charged ions hold the compound together.
Every solid liquid gas and plasma is composed of neutral or ionized atoms. Ferrum and atomic number 26. Web CBSE Class 10 Science MCQs Chapter 3 Metals and Non-Metals are available here for students practice.
Web In chemistry a nonmetal is a chemical element that generally lacks a predominance of metallic properties. Rank the following elements in order of decreasing atomic radius. Formally a string is a finite ordered sequence of characters such as letters digits or spaces.
Web Microsoft has responded to a list of concerns regarding its ongoing 68bn attempt to buy Activision Blizzard as raised by the UKs Competition and Markets Authority CMA and come up with an. Ionic bonding involves the transfer of valence electrons primarily between a metal and a nonmetal. Are usually crystalline solids made of ions Have high melting and boiling points.
Web When metals are next to each other the valence electrons dont just stay on their own atom. For example sodium chloride melts at 801 C and boils at 1413 C.

Which Of The Following Pairs Has The Same Size

7 Study Guide
Pyrimidopyridine Compound Used As A Csbp Rk P38 Modulator Patent 2436686

Complex Formation Of Niii Cuii Pdii And Coiii With 1 2 3 4 Tetraaminobutane Zimmer 2001 Chemistry A European Journal Wiley Online Library

Polyatomic Ions Common Polyatomic Ions Article Khan Academy

Wikipedia Talk Wikiproject Elements Archive 50 Wikipedia

Chemistry Archive November 04 2020 Chegg Com

Chapter 7 Ionic Compounds And Metals Chemistry Matter And Change Ppt Download
Aqa Bonding Structure And Properties L3 Covalent Bonding

7 2 Ionice Bons And Compounds Section Review Class Section Review 5 Objectives 0 Explain The Electrical Charge Of An Ionic Compound 0 Describe Course Hero

Dental Mastery Board Pdf Periodontology Dentistry Branches
Inorganics Free Full Text Smart Ligands For Efficient 3d 4d And 5d Metal Single Molecule Magnets And Single Ion Magnets
Chemistry Revision Notes 2012 Pdf
O Dpg Verhandlungen
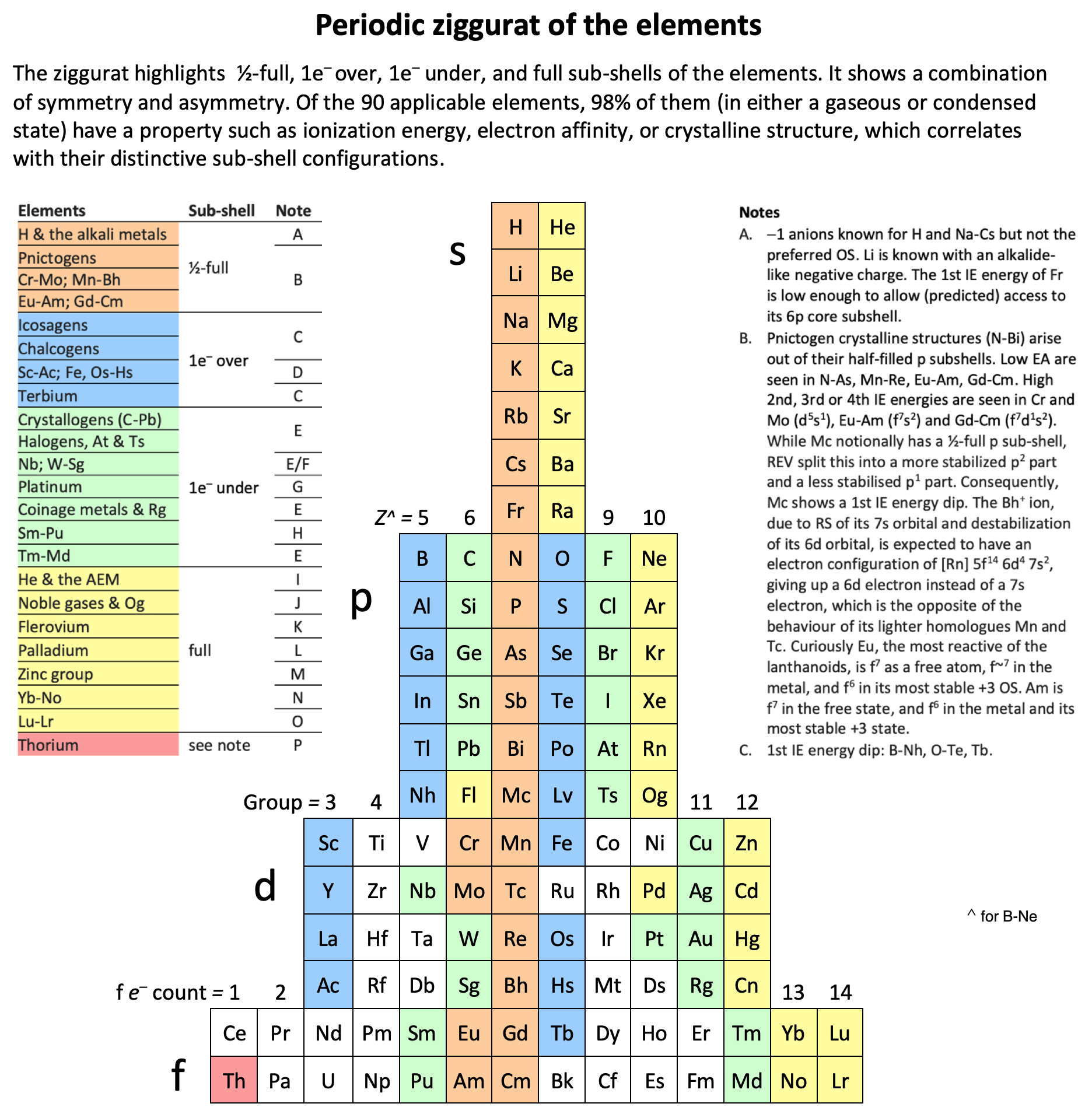
Internet Database Of Periodic Tables Chemogenesis

Naming Ionic Compounds Worksheet Easy Hard Science

Answers Ionic Bonding And Ionic Compounds Mutiple Choice 2 2012 07 05 Studocu